Cristcot’s Novel Rescue for UC Flares
The Company’s lead development product is at the end of Phase 3 clinical trials for patients living with ulcerative colitis (UC). With an endpoint of clinical remission defined by mucosal healing in just 4 weeks, the Cristcot drug is poised to be the only rescue drug for UC, for use alone or with other maintenance therapies.

Patient Stories
“Bleeding and uncontrolled bowel spasms are overwhelming. Rectal medications are a part of life for people with IBD—it’s the fastest relief from symptoms.”

UC’s Impact on Quality of Life
Globally, the majority of patients with moderate to severe disease reported UC to be mentally exhausting; even patients with milder UC or patients who self-reported being in remission agreed. Most patients felt that UC controlled their life rather than them controlling their UC, while approximately two-thirds of patients felt that they spent more time in the bathroom than anywhere else.
The majority of physicians thought that more than half of their patients with UC believed that bathroom urgency and spending significant time in the bathroom were part of living with UC.
Overall, the majority of patients agreed that having UC impacted their work, including those who considered themselves to be in remission.
76% of patients wished that they had more medication choices to treat their UC.

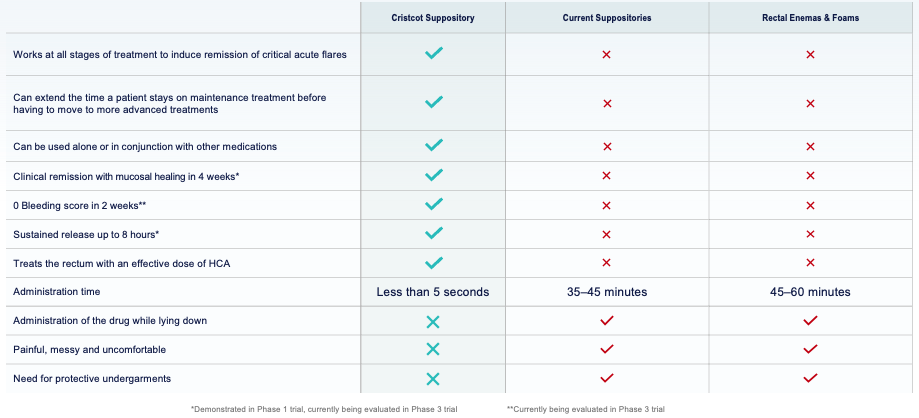
Cristcot’s Patented Suppository for the Treatment of UC
Large body of literature and clinical evidence shows hydrocortisone acetate (HCA) to be safe and effective in the treatment of UC. However, the compound has never been fully characterized or FDA approved in suppository dosage form.
A novel HCA suppository formulation to be delivered with our Sephure® suppository applicator, allows proper placement of medication above the anal-rectal line.
Ensures consistent drug delivery, eliminates medication leakage and the need for protective undergarments.
Unlike other suppositories, the Cristcot proprietary drug formulation has a rapid release profile for faster bioavailability, and the small dosing volume allows for ease of retention without medication leakage.
Patient Stories
All patients with UC have rectal disease involvement, and rectal symptoms are the most painful and debilitating.
“If my doctor told me that suppository medication would relieve symptoms of bathroom urgency and rectal bleeding, I would have no problem with it. I will do what it takes to have some relief and to feel better!”

Solves an Unmet Need in UC Treatment
Intellectual Property and Market Protections
Strong IP portfolio includes composition of matter and utility patents covering the drug formulation, devices, manufacturing processes, drug testing assay methods and trademarks, extending market exclusivity through late 2036
Regulatory market exclusivity of 3–5 years, with patents providing extended market exclusivity through late 2036
Unapproved hydrocortisone acetate suppositories will be removed from the market after approval of the Cristcot drug in accordance with the Hatch Waxman Act and FDA Code of Federal Regulations